Overview
|
The development and maintenance of neuronal networks is the basis for brain function. To develop and properly connect our intricate network of estimated 86 billion neurons requires precise regulation of wiring in time and space . Yet how neurons develop their individual morphology, find the right partners and maintain functional connectivity allowing us to learn and remember throughout life is still not well understood.
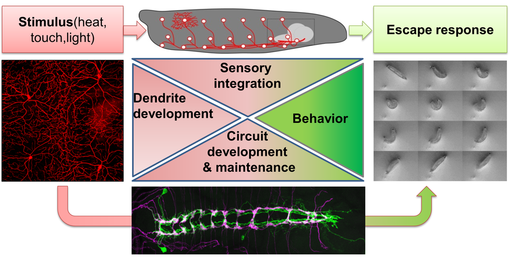
To get insight into these questions we use Drosophila melanogaster larvae as a model system. The approximately 12.000 functional neurons in the larval brain form a simple yet sufficiently complex nervous system to produce a range of behaviors supporting growth and survival, which we can study with high precision. The high degree of conserved molecular functions further allows us to deduce general principles of network formation and function and to extrapolate the results to higher organisms. In particular, we study the peripheral nervous system (PNS) and its circuitry. The PNS consists of regularly arranged sensory neurons lining the larval body wall. Each sensory neuron class features stereotyped morphology and specific functions, ranging from mechanosensation to nociception. |
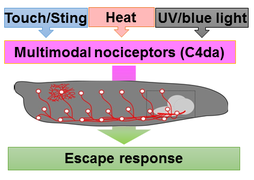
We currently focus our efforts on understanding the nociceptive network in this system, which detects noxious stimuli (e.g. heat and touch) resulting in a stereotyped escape response. Sensory dendrites of nociceptive neurons cover the entire larval body-wall and are also an excellent system to address basic mechanisms of dendrite development. The downstream network is surprisingly complex and we use it to study circuit development, maintenance and sensory integration at the molecular, cellular, functional and behavioral level.
|
Projects
Molecular mechanisms of dendrite patterning
|
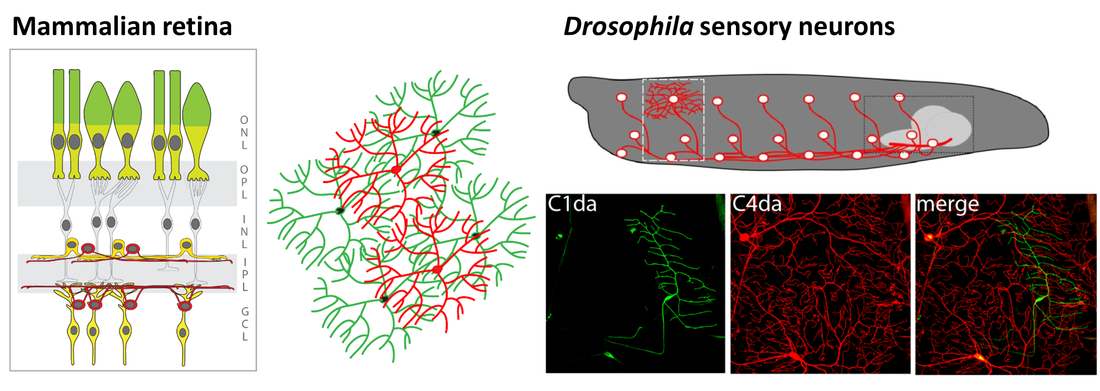
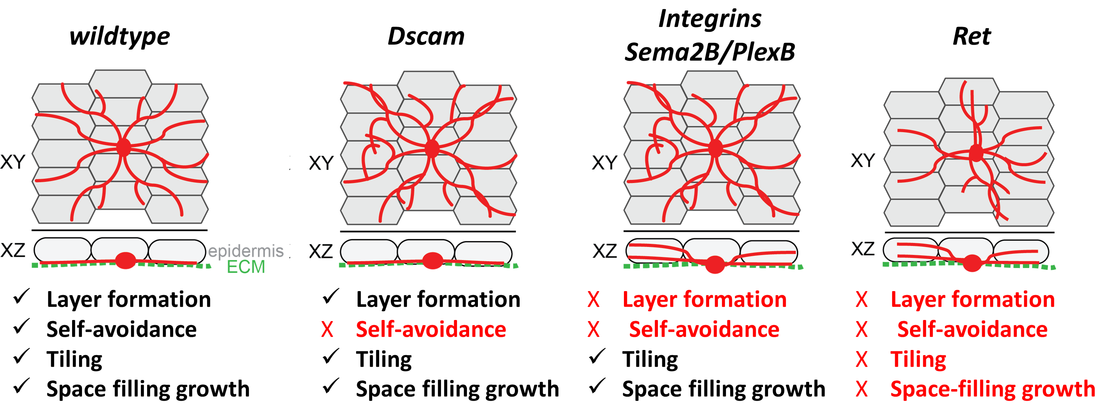
Figure 2: Organization of sensory neuron dendrites and identified molecular cues involved in basic patterning mechanisms . Sensory dendrites grow on an epidermal cell layer attached to an extracellular matrix (ECM) forming a 2-dimensional layer. This ensures self-avoidance (dendrites of the same neuron), tiling (no overlap of dendrites between neighboring neurons) and space-filling growth. We have contributed to several studies identifying molecules regulating these patterning mechanisms, including Dscam, Integrins, Sema2B/ PlexB, and Ret.
|
Work on larval sensory neuron development has significantly contributed to the identification of key molecules and mechanisms regulating basic dendrite patterning. These include Dscam, a cell surface receptor required for self-avoidance, as well as integrins and Semaphorin/Plexin, which mediate ECM adhesion and layer formation to ensure self-avoidance.
In our recent work we have identified the neuronal receptor tyrosine kinase Ret, which is required for the formation of space-filling dendritic fields of Drosophila nociceptive neurons. We are studying its upstream and downstream signals which promote and stabilize dendrite growth and adhesion. Ret function enables spreading of dendrites to establish complete receptive field coverage. These studies allow us to gain mechanistic insight into the basis of dendritic field development and patterning, which is essential for neuronal network function. |
Maintenance of circuit integrity and synaptic specificity
|
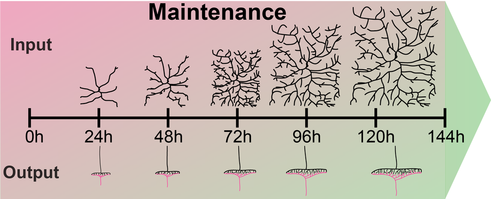
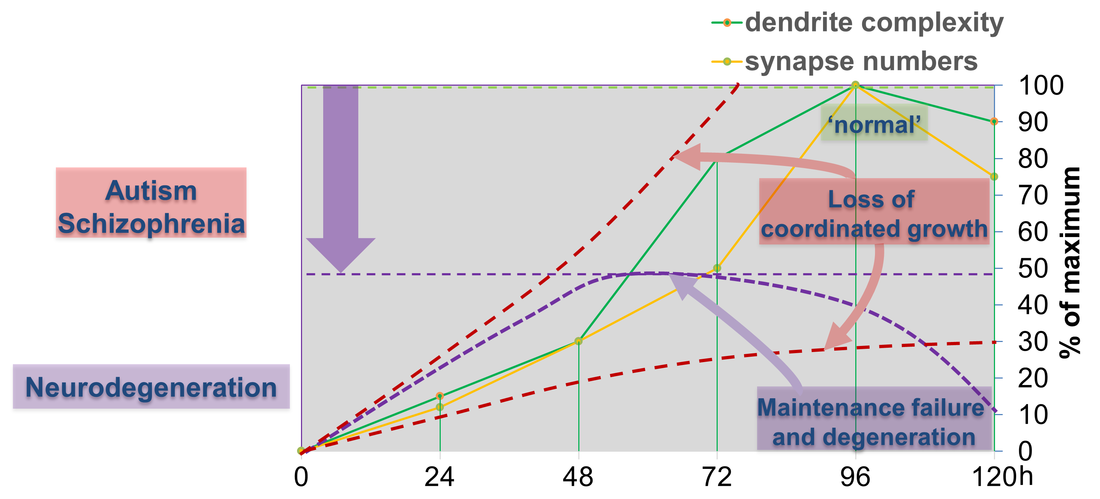
Figure 4: Dendritic and synaptic growth during larval development is highly regulated. Loss of coordinated growth due to aberrant cellular signals skews morphology and connectivity, which are hallmarks of neurodevelopmental disorders like autism and schizophrenia. Alternatively, failure to maintain developmental dendrite and synapse growth due to loss of gene function can result in growth arrest and eventually degeneration of synapses, axons and dendrites.
|
The first class is required for coordinated growth to keep neuronal and synaptic growth rates in sync with larval development. Genes regulating this process also seem to be required for circuit specific addition of synapses. We are investigating the underlying signaling machinery to gain mechanistic insight into the regulation of growth synchronization and synaptic specificity. This work is of particular relevance as abnormal connectivity has been linked to neurodevelopmental disorders including autism and schizophrenia.
We have also identified a second class of regulators, which are required to sustain neuronal morphology and synaptic connectivity of sensory neurons during larval development. The loss of these genes results in maintenance failure and degeneration suggesting a general failure of basic cellular functions underlying maintenance of neuronal morphology and connectivity. We investigate potentially common and diverging pathways to gain conceptual understanding of circuit maintenance, degeneration and its reversal. |
Neuromodulatory control of behavior
|
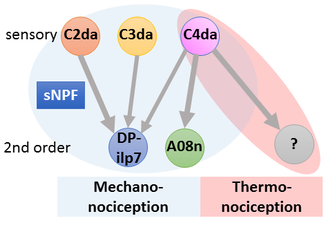
Circuits need to be functional to sustain behavior, and most larval behaviors are consistently maintained during development. This is particularly true for nociception, which is an evolutionary conserved mechanism to detect and avoid noxious stimuli. Similarly to vertebrates, Drosophila larval nociceptors are able to detect different modalities including nociceptive touch and heat. How behavioral responses to these distinct modalities are encoded at the network level has so far not been elucidated. We have recently uncovered a circuit and neuromodulatory mechanism encoding mechano-nociceptive escape behavior (Hu et al., Nat. Neurosci. 2017), which relies on the Drosophila Neuropeptide Y homolog sNPF. sNPF action on the identified circuit elements is specific to mechano-nocicieption, while thermo-nociception seems to be encoded by a different part of the network.
|
We are investigating how neuromodulation in this circuit regulates nociceptive responses using molecular, optogenetic and behavioral assays. Our studies aim to shed light on neuropeptide action in network function and behavioral action selection.
|